For example hydrogen in H 2 oxygen in O 2 nitrogen in N 2 carbon in diamond etc have oxidation numbers of zero. Oxidation occurs when the oxidation number of an atom becomes larger.

How To Find The Oxidation Number For N In No2 Nitrogen Dioxide Youtube
Chemistry The Central Science Chapter 22 Section 7

Oxidation State Of Amino Group Chemistry Stack Exchange
Likewise diimide which has two nitrogen atoms double-bonded to each other and its organic derivatives have nitrogen in the oxidation state of 1.

Oxidation number of nitrogen. Product yields mass prod namely NH 3 for N 2 reduction and NO 3 for N 2 oxidation relative to the size of the system from which nitrogen contaminants may originate mass sys are. It allows to control temperature during transportation and distribution with indirect liquid nitrogen injection. Compound Ions Oxidation No.
26 Facts about Nitrogen. According to the structure the symmetry suggests a -1 on each bridging sulfur colorblueblue just like the bridging O atoms in a peroxide and a 6 colorredred on each central sulfur like in sulfate. This means that the nitrogen atom must have an oxidation number of -3.
Oxidation number of oxygen is -2. Fluorine for example has the oxidation number 1 in all its compounds. Reduction occurs when the oxidation number of an atom becomes smaller.
The sum of the oxidation numbers of all the atoms in an ion or molecule is equal to its net charge. While fully ionic bonds are not found in nature many bonds exhibit strong ionicity making. With these facts about nitrogen let us learn about its chemistry physical properties atomic mass and much more.
The oxidation number of chlorine can be -1 0 1 3 4 5 or 7 depending on the substance containing the chlorine. Nitrogen preserves and protects foods with Modified Atmosphere Packaging MAP to minimize oxidation micro-organism growth or package collapse. Nitrogen nonmetallic element of Group 15 Va of the periodic table.
Other inorganic nitrogen compounds are nitric acid HNO 3 ammonia NH 3 the oxides NO NO 2 N 2 O 4 N 2 O cyanides CN- etc. The oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero. The oxidation state of carbon increases from 2 to 4 while the oxidation state of the hydrogen decreases from 1 to 0.
Identify the atoms which undergo change in oxidation number. Oxidation number from equation. Free radicals are produced by the reaction of unsaturated fatty acids with molecular oxygen and traces of other oxidants as metal ions Fe 3 and Cu 2.
Values are given for typical oxidation number and coordination. In hetero diatomic molecules all bonds formed between the atoms are considered as ionic. For example transition metals do not adhere to any fixed rules and tend to exhibit a wide range of.
We know in most occasions oxidation umber of oxygen is -2. 4 -3 1. Ii Heteronuclear diatomic molecule.
A corresponding compound for antimony is Sb 2 C 6 H 5 4 where the antimonys oxidation state is II. Nitrogen is a chemical element with atomic number seven and atomic weight 14. We know that the overall charge of the ammonium molecule is 1.
Ramis-Ramos in Encyclopedia of Food Sciences and Nutrition Second Edition 2003 Lipid Oxidation. For example nitrogen can have any oxidation number between 3 as in ammonia NH 3 and 5 as in nitric acid HNO 3. Since the oxidation number of nitrogen decreases from 5 to 4.
Keep in mind that oxidation states can change and this prediction method should only be used as a general guideline. Nitrogen in the form of ammonium chloride NH 4 Cl was known to the alchemists as sal ammonia. The oxidation number of an element in a monatomic ion is equal to the charge on that ion.
Take oxidation number of nitrogen is x. Redox reactions are comprised of two parts a reduced half and an oxidized half that always occur together. The above table can be used to conclude that boron a Group III element will typically have an oxidation state of 3 and nitrogen a group V element an oxidation state of -3.
Overall charge of N 2 O molecule is 0. The reduced half gains electrons and the oxidation number decreases while the oxidized half loses electrons and the oxidation number increases. Its symbol is N.
Electron affinity The energy released when an electron is added to the neutral atom and a negative ion is formed. Oxidation of sulfur dioxide by nitrogen dioxide accelerated at the interface of. Sodium nitrate NaNO 3 and potassium nitrate KNO 3 are formed by the decomposition of organic matter with compounds of these metals presentIn certain dry areas of the world these saltpeters are found in quantity and are used as fertilizers.
A partial electron transfer is a shift in the electron density near an atom as a result of a change in the other atoms to which it is covalently bonded. The most common oxidation numbers are -1 as. An oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reductionThe term oxidation state is often used interchangeably with oxidation number.
Others notably the nonmetals and the transition elements can assume a variety of oxidation numbers. To find the correct oxidation state of N in N2 Nitrogen gas and each element in the molecule we use a few rules and some simple mathFirst since the N2. The oxidation number of hydrogen or oxygen nitrogen chlorine in respective molecules is zero.
Write the oxidation number of each atom in the skeleton equation. The oxidation state 25 is just the average oxidation state for the S atom. Similarly realgar has arsenic-arsenic bonds so the arsenics oxidation state is II.
In ionic compounds the ionic charge of an atom is its oxidation number. Some of oxidation numbers of each element in the molecule should equal to the zero. For example the oxidation number of chlorine in Cl2 phosphorus in P4 and sulfur in S8 is 0.
Certain elements assume the same oxidation number in different compounds. It has a melting point of 20986 C 3458 F and a boiling point of 1958 C 3204 F. The SMPS captured approximately 6687 of the total particle number based on a log.
X2 -2 0. Its atomic number is 7 and it is denoted by the symbol N in the periodic table. There are two nitrogen atoms in N 2 O.
It is a colorless odorless tasteless gas that is the most plentiful element in Earths atmosphere and is a constituent of all living matter. Oxidation synonyms oxidation pronunciation oxidation translation English dictionary definition of oxidation. Calculate the increase and decrease in oxidation number wrt reactant atoms.
Oxidation and reduction are therefore best defined as follows. Lipid oxidation is a process that results in rancidity and deterioration of fats and progresses via free-radical propagated chain reactions. Nitrogen is used to chill freeze or control temperature of food products.

How To Find The Oxidation Number For N In N2 Nitrogen Gas Youtube

How Can Nitrogen Exhibit 4 Oxidation State Quora

In Which Structure Does Nitrogen Have The Lowest Oxidation N Clutch Prep

Suggest A List Of The Substances Where Carbon Can Exhibit Oxidation States From 4 To 4 And Nitrogen From 3 To 5 From Chemistry Redox Reactions Class 11 Assam Board

Nitrogen Species And Their Oxidation States Download Table
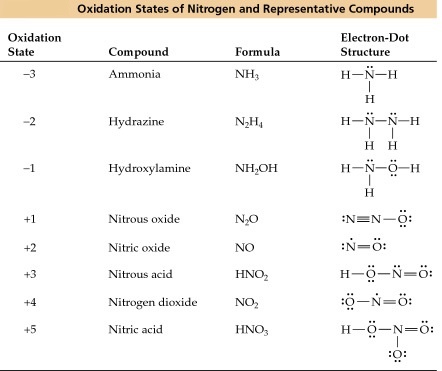
Oxidation Numbers Of Nitrogen In Some Of Its Compounds Science Online

Pdf Compounds With Various Oxidation States Of Nitrogen Nitrosyl Compound Ammonia Semantic Scholar

Oxidation Number Of Nitrogen In No2 Nitrogen Dioxide
